how many protons do lithium have|Chemistry of Lithium (Z=3) : Pilipinas The alkali metals are also called the lithium family, after its leading element. Like the other alkali metals (which are sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr)), lithium has a single valence electron that, in the presence of solvents, is easily released to form Li . Because of this, lithium is a good conductor of heat and electricity as well as a highly reactive element, tho. 3D Porn Comics Read Online Download Free In PDF Format With High Quality Images. All 3D Comic Episodes With Latest Update.Use Google Flights to plan your next trip and find cheap one way or round trip flights from General Santos City to Cebu City. Find the best flights fast, track prices, and book with confidence.
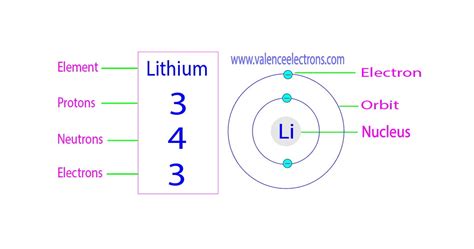
how many protons do lithium have,Lithium is a chemical element with atomic number 3 which means there are 3 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e .Atomic Number – Protons, Electrons and Neutrons in Lithium. Lithium is a .
Element Lithium (Li), Group 1, Atomic Number 3, s-block, Mass 6.94. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. Jump to main contentThe alkali metals are also called the lithium family, after its leading element. Like the other alkali metals (which are sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr)), lithium has a single valence electron that, in the presence of solvents, is easily released to form Li . Because of this, lithium is a good conductor of heat and electricity as well as a highly reactive element, tho.Lithium is the 3rd element in the periodic table and has a symbol of Li and atomic number of 3. It has an atomic weight of 6.940 and a mass number of 7. Lithium has three protons and four .
how many protons do lithium have Wayne Breslyn. 792K subscribers. 310. 36K views 4 years ago. In this video we’ll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for .

More recently lithium has become important in dry-cell batteries and nuclear reactors. Some compounds of lithium have been used to treat manic depressives. Lithium is . Unlocking the Power: The Protons of Lithium 👉 The Power of Lithium 👉 Discover the atomic wonders of lithium as we explore its protons and their impact on i.Overview. Lithium is the first member of the alkali metal family. The alkali metals are the elements that make up Group 1 (IA) of the periodic table. The periodic table is a chart that shows how chemical elements are related to one another. .Atomic Number – Protons, Electrons and Neutrons in Lithium. Lithium is a chemical element with atomic number 3 which means there are 3 protons in its nucleus. Total number of protons in the nucleus is called the atomic number .Steric data. Atomic radius: 2.05 Å Ionic radius: 0.76 Å (+1) Covalent radius: 1.23 Å Atomic volume: 13.10 cm³/mol Density (293 K): 0.53 g/cm³ Crystal structure: cubic body centered. Isotopes. .
The number of protons in the nucleus of an atom is its atomic number (\(Z\)). This is the defining trait of an element: Its value determines the identity of the atom. For example, any atom that contains six protons is the .Solution. A lithium atom contains 3 protons in its nucleus irrespective of the number of neutrons or electrons. a. \[ \begin{align}\text{atomic number} = \left( \text .The Dipole Polarizability of Lithium is 164.1125 plus or minus 0.0005 a₀. Li has a C6 Dispersion Coefficient (CD) of 1392 a₀, and C6 Dispersion Coefficient (GB) of 1410 a₀. The Allotropes of Element 3 is . The Neutron Cross Section of Lithium (Li) is 71. The Quantum Numbers of Lithium is 2S1/2. The Space Group of Li is 229 (Im_3m). Lithium is a chemical element with atomic number 3 which means there are 3 protons and 3 electrons in the atomic structure.The chemical symbol for Lithium is Li. It is a soft, silvery-white alkali metal. Under standard conditions, it is the lightest metal and the lightest solid element. Like all alkali metals, lithium is highly reactive and flammable, and is stored in .
The diameter of an atom of hydrogen is 0.1nm (1.0nm = 10-9 m). So, if 1000 crore atoms of hydrogen are arranged side by side, it will be 1 meter long. However, it has been possible to detect atoms by increasing the vision of a very powerful electron microscope by .Watch this video to learn how protons, neutrons, and electrons are arranged in atoms, and how the number and distribution of these subatomic particles determine the identity and properties of elements. This is a .

How many protons will lithium have if the mass number is 8? Lithium with a mass number of 8 will have 3 protons. The mass number represents the total number of protons and neutrons in an atom's .
A proton is one of the subatomic particles that make up matter. In the universe, protons are abundant, making up about half of all visible matter.It has a positive electric charge (+1e) and a rest mass equal to 1.67262 × 10 −27 kg (938.272 MeV/c 2)— marginally lighter than that of the neutron but nearly 1836 times greater than that of the electron.
Lithium-6 - isotopic data and properties - ChemLin
For example, how many protons does Ca have? For this, you just need to look up the atomic number of Ca, which we find, from the periodic table, that it is 20. Therefore, Ca has 20 protons. Do not get confused if the element is given with some other data such as the atomic mass or the average atomic mass. The answer is the protons and the electrons. The protons have a positive charge, while electrons are negatively charged.If you remove one or more electrons from the atom, you will receive a positive ion called a cation as you will leave more protons than electrons. Similarly, by adding electrons, you will gain a negative ion, called an anion. Normally, you .
how many protons do lithium have Chemistry of Lithium (Z=3) Watch this video to learn how to define and compare the atomic number, mass number, and isotopes of atoms. Discover how these concepts are related to the structure and behavior of atoms, and how .
Niels Bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells. . the group 1 elements, including hydrogen (H), lithium (Li), and sodium (Na), .A proton is one of the subatomic particles that make up matter. In the universe, protons are abundant, making up about half of all visible matter.It has a positive electric charge (+1e) and a rest mass equal to 1.67262 × 10 −27 kg (938.272 MeV/c 2)— marginally lighter than that of the neutron but nearly 1836 times greater than that of the electron.Isotopic Distributions. Although carbon-12 is the most abundant type of isotope in carbon, it is not the only isotope. In addition to 12 C, a typical sample of carbon contains 1.11% 13 C, with 7 neutrons and 6 protons, and a trace 14 C, with 8 neutrons and 6 protons. The nucleus of 14 C is not stable, however, but undergoes a slow radioactive decay that is the basis of the carbon-14 .If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
Protons and Neutrons in Beryllium. Beryllium is a chemical element with atomic number 4 which means there are 4 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z.The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.
How many protons does a beryllium atom have? Protons are the permanent core particles of an atom. It resides in the center or nucleus of the atom. When a hydrogen atom removes an electron from its orbit, the positively charged particle that remains is called proton. Hence, the proton is expressed by H +.
how many protons do lithium have|Chemistry of Lithium (Z=3)
PH0 · Lithium (Li) [3] — Chemical Element — Periodic Table
PH1 · Lithium (Li)
PH2 · Lithium
PH3 · LITHIUM
PH4 · How to find the Number of Protons, Electrons, Neutrons for
PH5 · How many protons does lithium have?
PH6 · Chemistry of Lithium (Z=3)